- Miscellaneous
- Protective Effect of Delta-Like 1 Homolog Against Muscular Atrophy in a Mouse Model
-
Ji Young Lee, Minyoung Lee, Dong-Hee Lee, Yong-ho Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha
-
Endocrinol Metab. 2022;37(4):684-697. Published online August 29, 2022
-
DOI: https://doi.org/10.3803/EnM.2022.1446
-
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material PubReader PubReader  ePub ePub
- Background
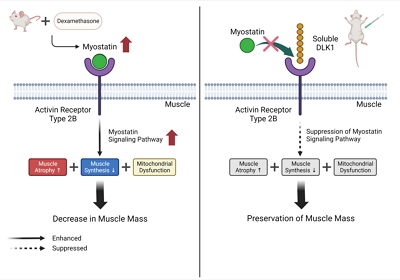
Muscle atrophy is caused by an imbalance between muscle growth and wasting. Delta-like 1 homolog (DLK1), a protein that modulates adipogenesis and muscle development, is a crucial regulator of myogenic programming. Thus, we investigated the effect of exogenous DLK1 on muscular atrophy.
Methods
We used muscular atrophy mouse model induced by dexamethasone (Dex). The mice were randomly divided into three groups: (1) control group, (2) Dex-induced muscle atrophy group, and (3) Dex-induced muscle atrophy group treated with DLK1. The effects of DLK1 were also investigated in an in vitro model using C2C12 myotubes.
Results
Dex-induced muscular atrophy in mice was associated with increased expression of muscle atrophy markers and decreased expression of muscle differentiation markers, while DLK1 treatment attenuated these degenerative changes together with reduced expression of the muscle growth inhibitor, myostatin. In addition, electron microscopy revealed that DLK1 treatment improved mitochondrial dynamics in the Dex-induced atrophy model. In the in vitro model of muscle atrophy, normalized expression of muscle differentiation markers by DLK1 treatment was mitigated by myostatin knockdown, implying that DLK1 attenuates muscle atrophy through the myostatin pathway.
Conclusion
DLK1 treatment inhibited muscular atrophy by suppressing myostatin-driven signaling and improving mitochondrial biogenesis. Thus, DLK1 might be a promising candidate to treat sarcopenia, characterized by muscle atrophy and degeneration.
- Endocrine Research
- Mechanism of Lipid Accumulation through PAR2 Signaling in Diabetic Male Mice
-
Dae Hyun Kim, Ye Ra Kim, EunJin Bang, Sugyeong Ha, Sang Gyun Noh, Byeong Moo Kim, Seong Ho Jeong, Hee Jin Jung, Ji Young Lee, Hae Young Chung
-
Endocrinol Metab. 2021;36(1):171-184. Published online February 24, 2021
-
DOI: https://doi.org/10.3803/EnM.2020.850
-
-
4,612
View
-
135
Download
-
2
Web of Science
-
2
Crossref
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material PubReader PubReader  ePub ePub
- Background
Protease-activated protein-2 (PAR2) has been reported to regulate hepatic insulin resistance condition in type 2 diabetes mice. However, the mechanism of lipid metabolism through PAR2 in obesity mice have not yet been examined. In liver, Forkhead box O1 (FoxO1) activity induces peroxisome proliferator-activated receptor γ (PPARγ), leading to accumulation of lipids and hyperlipidemia. Hyperlipidemia significantly influence hepatic steatoses, but the mechanisms underlying PAR2 signaling are complex and have not yet been elucidated.
Methods
To examine the modulatory action of FoxO1 and its altered interaction with PPARγ, we utilized db/db mice and PAR2-knockout (KO) mice administered with high-fat diet (HFD).
Results
Here, we demonstrated that PAR2 was overexpressed and regulated downstream gene expressions in db/db but not in db+ mice. The interaction between PAR2/β-arrestin and Akt was also greater in db/db mice. The Akt inhibition increased FoxO1 activity and subsequently PPARγ gene in the livers that led to hepatic lipid accumulation. Our data showed that FoxO1 was negatively controlled by Akt signaling and consequently, the activity of a major lipogenesis-associated transcription factors such as PPARγ increased, leading to hepatic lipid accumulation through the PAR2 pathway under hyperglycemic conditions in mice. Furthermore, the association between PPARγ and FoxO1 was increased in hepatic steatosis condition in db/db mice. However, HFD-fed PAR2-KO mice showed suppressed FoxO1-induced hepatic lipid accumulation compared with HFD-fed control groups.
Conclusion
Collectively, our results provide evidence that the interaction of FoxO1 with PPARγ promotes hepatic steatosis in mice. This might be due to defects in PAR2/β-arrestin-mediated Akt signaling in diabetic and HFD-fed mice.
-
Citations
Citations to this article as recorded by  - Biochanin‐A has antidiabetic, antihyperlipidemic, antioxidant, and protective effects on diabetic nephropathy via suppression of TGF‐β1 and PAR‐2 genes expression in kidney tissues of STZ‐induced diabetic rats
Jamal Amri, Mona Alaee, Rasool Babaei, Zahra Salemi, Reza Meshkani, Ali Ghazavi, Ahmad Akbari, Mehdi Salehi
Biotechnology and Applied Biochemistry.2022; 69(5): 2112. CrossRef - Delineation of the healthy rabbit liver by immunohistochemistry – A technical note
Gabriella Meier Bürgisser, Olivera Evrova, Dorothea M. Heuberger, Julia Rieber, Pietro Giovanoli, Maurizio Calcagni, Johanna Buschmann
Acta Histochemica.2021; 123(7): 151795. CrossRef
- Involvement of Polyamine in Growth Hormine Secretion from the GH3 Cells.
-
Ji Young Lee, Byoung Ki Kim
-
J Korean Endocr Soc. 1998;13(3):313-323. Published online January 1, 2001
-
-
-
 Abstract Abstract
 PDF PDF
- BACKGROUND
S: Polyamines are known to be essential for cell growth and differentiation. Recently, possible roles of the polyamine in signal transduction as neurotransmitter, modulator, or second messenger are suggested in many studies. Furthermore, it is widely studied that possible roles of polyamine are involved in the action of hormone. Thus, it was to investigate the effect of polyamines in the cell proliferation and secretion of GH from the GH cells. METHODS: Cells(5*10 cells/mL) were incubated for 3 days in DMEM containing test drugs and labeled with 20pCi/mL of [S]-methionine for 2 hr. Proteins secreted into the medium were separated by 13% SDS-gel electrophoresis, then autoradiography was performed to identify radiolabeled proteins. [S]-methionine labelled GH was identified by radioimmuno-precipitation. Total protein synthesis was determined from the radioactivity of the cell homogenate by liquid scintillation counter. The intracellular polyamine content was determined by HPLC. RESULTS: Externally added polyamines(putrescine, spermidine, spermine) induced cell proliferation in a dose-dependent manner at proper concentrations, specifically 50pM putrescine increased GH secretion, DFMO or MGBG, which is polyamine biosynthetic inhibitor, inhibited GH secretion in a dose-dependent fashion, In the cells treated with 20mM or 0.01mM MGBG, total protein synthesis were decreased only to 90 or 76% of the control levels and cell proliferation was also slightly inhibited. However the secretion of GH was severely blocked to 37% or 35% of the control. Hydrocortisone at 5 pM stimulated the secretion of GH to 153% of basal secretion, also doubled intracellular putrescine content. CONCLUSION: The present data show that externally added polyamines induced cell proliferation and GH secretion. Also, extemally added putrescine stimulated GH secretion significantly. GH secretion was inhibited by polyamine metabolic inhibitor in a dose-dependent manner and polyamine metabolic inhibitors, at proper concentrations, specifically blocked GH secretion without any significant influence on the total protein synthesis. The above results imply the involvement of polyamine in GH secretion.
|